Over the past few years, the area of oncology has moved to precision medicine, the ability to target particular molecular pathways that promote the growth of cancer cells. The ATR kinase (Ataxia Telangiectasia and Rad3-related protein) which is one of the major regulators of the response of the cell to DNA damage is among the most promising targets.
ATR signaling is very critical in the survival of cancer cells, which in most cases are prone to genomic instability and replication stress. ATR inhibition is an attractive treatment option due to this dependency. Berzosertib (VE-822) ATR inhibitor, is one of the best in this category with encouraging preclinical activity and an early clinical trial.
What is ATR and How Its Role in DNA Damage Response
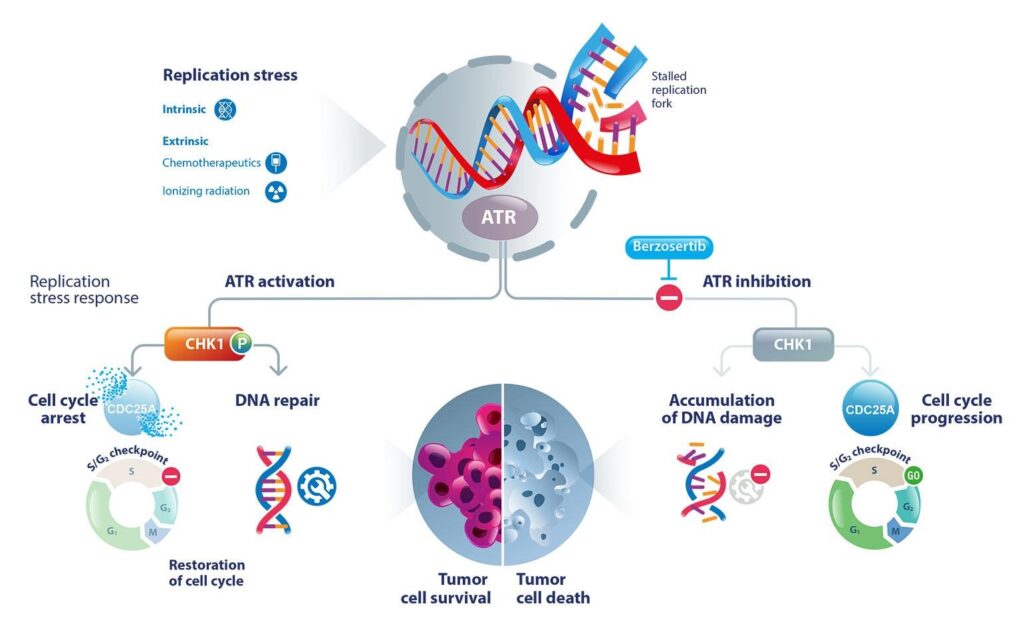
ATR is a serine/threonine kinase which is key in conserving the stability of the genome. It comes on when single-stranded DNA regions are present, which occur during replication stress, which is also a typical phenomenon of fast-dividing cancer cells. As a consequence of activation, ATR phosphorylates a number of downstream targets, including CHK1 (Checkpoint Kinase 1), which arrests the cell cycle and permits time to repair the DNA.
This is an essential checkpoint mechanism that is necessary to avoid destructive genomic damage. But in the cancer cells which already have dysfunctional p53 or ATM (Ataxia Telangiectasia Mutated) pathways, ATR is even more important.
Blocking ATR means that the damaging effect of the cancer cells is further triggered by drugs such as Berzosertib, which force the cancer cells to undergo apoptosis. A key principle of contemporary targeted therapy is this principle of synthetic lethality, wherein two genetic or molecular defects interact to lethally affect the cell.
The Development of Berzosertib (VE-822)
The development code for Berzosertib was VE-822, and it is a small-molecule inhibitor of ATR kinase that is both powerful and selective. After its development by Vertex Pharmaceuticals, Merck KGaA and other collaborators moved it forward for clinical trials.
In order to increase the effectiveness of DNA-damaging medicines like cisplatin, gemcitabine, and topoisomerase inhibitors against tumors, preclinical research showed that Berzosertib could make tumor cells more sensitive to these treatments.
Its remarkable selectivity for ATR over related kinases like ATM and DNA-PK was demonstrated in in vitro experiments, reducing the likelihood of off-target effects. The drug’s impressive synergy with radiation therapy makes it a promising option for solid tumors, a treatment modality that often includes radiation.
Mechanism of Action
The kinase activity of ATR is inhibited when Berzosertib binds to its ATP-binding site. This blockage stops CHK1 and other DNA damage repair downstream effectors from being phosphorylated. The accumulation of unrepaired lesions and the collapse of replication forks lead to cell death.
The effect of inhibiting ATR is minimal in normally functioning cells due to their reduced replication stress and undamaged DNA repair machinery. Cancer cells, on the other hand, are incredibly vulnerable due to their oncogene-driven proliferation and impaired checkpoint mechanisms. Because of this bias, Berzosertib is able to selectively target cancer cells while avoiding healthy ones.
Research and Clinical Trials
Berzosertib has shown promising results according to https://www.nature.com/articles/s41416-021-01405-x in terms of safety, tolerability, and effectiveness in early-phase clinical trials. Both monotherapy and combination with standard chemotherapeutic drugs were assessed in a Phase I trial (NCT02157792) involving Berzosertib. The results demonstrated tolerable toxicity profiles, with nausea, exhaustion, and hematologic consequences such anemia and neutropenia being the most prevalent side effects.
The combination of Berzosertib and gemcitabine, a nucleoside analog that causes replication stress, showed especially encouraging results. Ovarian, pancreatic, and small-cell lung cancers were among the solid tumor types that were affected by the combination’s synergistic anti-tumor activities.
Since ATM-deficient malignancies rely heavily on ATR signaling and lack one of the main DNA repair mechanisms, additional research investigated the use of Berzosertib in these tumors. The results provide support for the idea of using biomarkers to guide patient selection, as they indicate that in some genetic situations, ATR inhibition may have an even greater impact.
The combination of chemotherapy and PARP inhibitors, another family of medications that target the DNA damage response, is another area of investigation for Berzosertib. Even in malignancies that are resistant to PARP medicines alone, preclinical data suggests that co-targeting PARP and ATR can elicit significant DNA damage and cell death.
Possible Benefits and Uses
Berzosertib’s main selling point is that it works well with other cancer treatments and is quite selective. It allows medications that cause DNA damage to work more effectively without significantly raising systemic toxicity by taking advantage of flaws in cancer cell biology. This enables doctors to improve the efficacy of treatments while ensuring manageable side effects.
Additionally, there is hope for overcoming therapeutic resistance with ATR inhibitors such as Berzosertib. Through improved repair mechanisms, many malignancies become resistant to substances that damage DNA. The chemo drug Berzosertib can make cancers more resistant to standard treatments by preventing ATR-mediated repair.
Combining Berzosertib with immunotherapy is another potential approach. The inactivation of ATR leads to DNA damage, which in turn increases the mutational burden of tumors and activates the immune system, which may improve responses to checkpoint inhibitors like anti-PD-1 or anti-PD-L1 antibodies. Although this is still a developing field, preliminary studies have shown synergistic potential that may reshape how refractory malignancies are treated.
Obstacles and Ways Forward
Despite the potential benefits, Berzosertib encounters a number of obstacles. Excessive suppression of ATR could affect normal proliferating cells, especially in bone marrow and gastrointestinal tissue, therefore finding the correct dose schedule is critical. individuals with ATM loss, BRCA mutations, or high replication stress signatures seem to be prime candidates; however, identifying accurate biomarkers is also necessary for selecting the individuals most likely to benefit.
With prolonged use, resistance mechanisms can also develop. Compensatory activation of alternate repair pathways may mitigate the consequences of ATR suppression, according to some research. To get around this resistance and have the reaction last longer, try taking Berzosertib with an inhibitor of a comparable target, like CHK1, PARP, or WEE1.
Researchers are currently concentrating on improving combination tactics, increasing the indications for ATR inhibition beyond solid tumors, and incorporating it into personalized medicine frameworks as clinical trials move forward.

